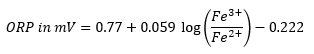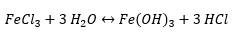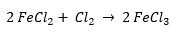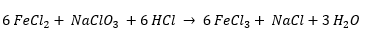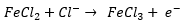Ferric chloride (FeCl3) is perhaps the most versatile of all metal-etching solutions. This is due to its capability of etching many different metals such as stainless steel, carbon steel, nickel & nickel-iron alloy, and copper alloy. On top of providing you the ability to etch different metals, it is also considered fairly economic. The main reason for that is because of its regenerative properties that allow you to etch more metal over time. If you are someone looking to start a chemical milling shop, on any scale, understanding ferric chloride regeneration can be a big game-changer for your process.
Why Ferric Chloride Regeneration?
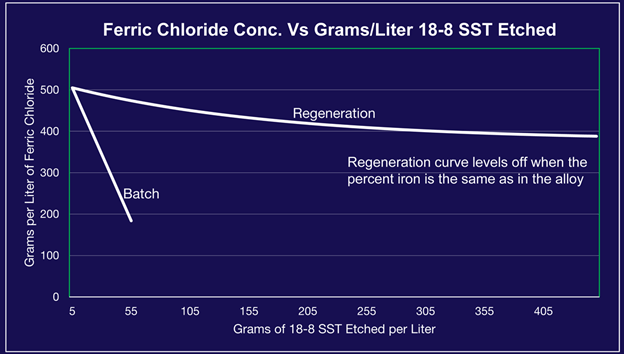
When FeCl3 etches metal, it becomes ferrous chloride (FeCl2). As you etch more metals and deplete your FeCl3, you will notice a reduction in etch rate. If you run a batch operation, where you use your etchant without making additions to assist the etching, you will notice a steady decline in the amount of metal you can dissolve (as shown in the graph above). If you utilize regeneration, where you convert FeCl2 back into FeCl3, you can effectively increase the amount of metal you can dissolve – thus allowing you to maintain a steadier etch rate over time and get more use out of your etchant.
Necessary Controls
There are a handful of different ways you can regenerate your ferric chloride, but before you consider going with regeneration, you will need certain basic controls to maintain your etchant’s quality. These controls will also help with the regeneration process by giving you indicators of when to add new reagents into your bath to start the regeneration reactions. The main parameters you will need to monitor with your ferric chloride etchant are free acid content, oxidation-reduction potential (ORP), and specific gravity.
Specific Gravity
Specific gravity is an etchant parameter that can affect etch rate. This is because specific gravity represents the amount of metal dissolved in the solution. Keeping the amount of metal dissolved in the solution constant helps maintain etch rate and surface roughness. Since the etching process adds more metal into the solution, you will normally see a rise in specific gravity as you etch. Having a specific gravity control can provide an indication of when it starts to get too high. Once specific gravity gets too high, it can easily be brought back down with the addition of water.
Controls for specific gravity can simply be done with a hydrometer. With the hydrometer, you can either make manual water adds when you notice the specific gravity gets too high, or you can rig it with a sensor so that water can be added automatically.
ORP
ORP, to simply put it, is a measurement of the FeCl3 to FeCl2 ratio. Whenever you use an ORP probe, the value you will be given is provided in mV. This is a result of the following equation:
Normally we recommend an ORP range of 580 to 605 mv for ferric chloride. Overall, the idea you should keep in mind with ORP is that higher values indicate you have more FeCl3 ready for the reaction. We recommend a max of 605 mv because beyond that point you can receive a reaction that is harder to control. If your ORP is too high, you can just run an iron panel through the machine until you get to a reasonable ORP value. If your ORP is low, then that is a sign that you need to regenerate because there is more FeCl2 that is slowing down your reaction.
Controls for ORP are usually done with two electrodes, one being platinum and the other being a silver-silver chloride reference electrode. Values extracted from these probes can then provide an indicator to you or your machine to initiate a regeneration process once ORP is too low.
Free acid
Free acid in a ferric chloride bath refers to the amount of hydrochloric acid (HCl) in your etchant. This is important because it makes the etching process more practical by increasing the etch rate. Although that is the main purpose, it also serves to extend the life of your etchant. It does this by preventing the following reaction:
Since this reaction is reversible, it will encourage the ferric chloride to stay in the solution and not react with water to create the insoluble ferric hydroxide (Fe(OH)3) by-product. Once your ferric chloride reacts to form ferrous hydroxide, there is no good way to convert it back to ferric chloride. Therefore, you are stuck with sediment in your etchant which can cause abrasions on your product and your etchant’s capacity to dissolve iron becomes reduced.
This parameter is perhaps the most difficult parameter of the 3 to control, and generally, the best way to know where your free acid content is at is to perform titrations as frequently as once every shift. It is possible to use pH for controlling free acid, but often it needs very frequent maintenance and calibration to work effectively.
A simpler way to keep free acid content consistent is to introduce a board counter as a control. If you know how much free acid you lose per panel you etch, you can get a rough idea of how much acid add back in. However, most HCl is lost through evaporation– therefore the board counter is best with continuous production. If you leave your etchant to set for a weekend, it may need checking when you return. No matter which way you try to control free acid, scheduled titrations are necessary for keeping the system in check.
Regeneration Options
Once you have established some controls for your ferric chloride etchant, you can start considering the different methods used for regeneration. The 3 main practical regeneration reactions are performed with chlorine gas, sodium chlorate, and electrolysis.
Chlorine Gas Regeneration
Chlorine gas (Cl2) can be used to regenerate ferric chloride by undergoing the following reaction:
This option is the most efficient way to regenerate ferric chloride. That is because there is very little heat that evolves from the reaction and there are no by-products that can accumulate in the bath. Although it is effective, there is often hesitation to use it because of the safety regulations and concerns that come with working around chlorine gas.
If you can manage the safety requirements that come with using chlorine gas, you can reduce the cost of etching by a factor of 9.[1]
Sodium Chlorate Regeneration
Sodium chlorate (NaClO3) can also be used to regenerate your ferric chloride etchant by undergoing the following reaction:
Using sodium chlorate is the next best way to perform regeneration because it only requires a simple pump feed of sodium chlorate and hydrochloric acid into your bath. The reaction is quick so making fast adjustments to your bath can be easily done.
The main downside to using sodium chlorate regeneration is the sodium chloride (NaCl) byproduct. As you etch and regenerate your ferric chloride with sodium chlorate, you will accumulate sodium chloride crystals in your bath. These crystals can create abrasions on your product and damage machine parts. Because of the crystallization, etching machines will need to be emptied and cleaned at regular intervals. One way to reduce the build-up of crystals is to make additions of fresh ferric chloride into the bath.
If you choose to go with sodium chlorate regeneration, you can reduce the cost of etching by a factor of 4.[1]
Electrolytic Regeneration
In electrolytic regeneration, the source of chlorine comes from hydrochloric acid. The reaction goes as follows:
This reaction involves using an anode, a cathode, and a special chloride ion-permeable membrane. On the anode side, is where you will have your HCl. The chloride ions get pulled through the membrane to meet the etchant for regeneration. What is nice about this method is that you can regenerate ferric chloride without having to introduce any new chemicals into your process.
The main downside of this process is that it comes at a high initial cost to create the cell and that the cell requires a significant amount of floor space. Even with the cost and space requirement put aside, regeneration by this method is slow. Because of the slow regeneration rate, the cell is often run overnight to completely regenerate the etchant.
If you have the floor space and available funds to invest in a sufficient electrolytic regeneration system, you can get a return on your investment in a range of 2 to 4 years. [2] [3]
Final Set-Up
Once you decide what regeneration process you would like to go with, feel free to contact us to help implement your regeneration procedure. Whether you are receiving a new piece of Chemcut equipment or already have a machine, we can provide you with the necessary steps to implement regeneration into your ferric chloride etcher.